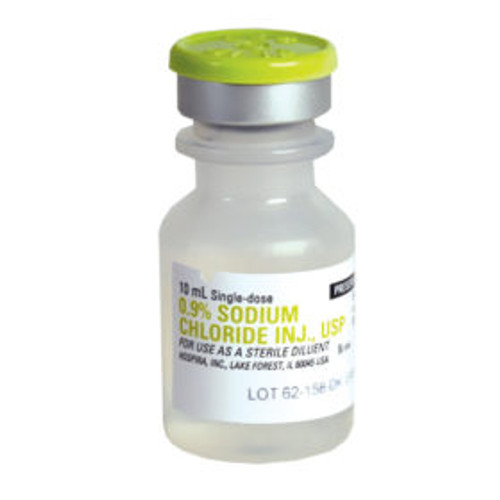
Product Description
Features
- Flip-top plastic vial
- This parenteral preparation is indicated only for diluting or dissolving drugs for intravenous, intramuscular or subcutaneous injection, according to instructions of the manufacturer of the drug to be administered
- The semi-rigid vial is fabricated from a specially formulated polyolefin
HOS 4888-010 CS/25 SODIUM CHOLRIDE, NACL, 10ML VIAL
Product Specifications
| Manufacturer # | 00409488810 |
| Manufacturer | Hospira |
| Application | Diluent |
| Container Type | Single Dose Vial |
| Dosage Form | Solution |
| Generic Drug Code | 03034 |
| Generic Drug Name | Sodium Chloride, Preservative Free |
| NDC Number | 00409-4888-10 |
| Storage Requirements | USP Controlled Room Temperature |
| Strength | 0.9% |
| Type | Intramuscular, Intravenous, or Subcutaneous |
| Volume | 10 mL |
| Latex Free Indicator | Not Made with Natural Rubber Latex |